This procedure applies to the Executive in their role of managing and administering the ESCC Non-conformance Control System. It also applies to the CTB with regard to their management of the ESCC AQP, to the SCSB for policy issues and to ESA as the Qualification Authority.
- Supplier Non Conformance Procedure
- Non Conformance Procedure Food Industry
- Non Conformance Procedure Template
- Non Conformance Procedure Sample
A non-conformance (or 'nonconformity') means that something went wrong.
- This procedure outlines the process used in the company for managing of non-conformance, corrective actions and preventive actions. SCOPE This procedure is applicable for handling of both internal and external non-conformances, and the corrective action request arising from customer complaints and system non-conformance.
- The non-conformance could be in a service, a product, a process, goods from a supplier, or in the management system itself. It occurs when something does not meet the specifications or requirements in some way. These requirements might be defined by the customer, a regulatory body, or in the internal procedures of the company.
The non-conformance could be in a service, a product, a process, goods from a supplier, or in the management system itself. It occurs when something does not meet the specifications or requirements in some way. These requirements might be defined by the customer, a regulatory body, or in the internal procedures of the company.
Here are some examples:
- You manufacture steel plates with a hole of size 10mm, but on inspection the hole measures 9mm.
- You supplied product to a customer that was not the colour they ordered.
- As a labour hire company you sent a worker to a client without the correct induction.
- Your consulting office sent a report to a client that is missing a section of work
- Your testing company misplaced some client samples
- A supplier has sent you the wrong product
- Your batch records are missing temperature information and the supervisor's signature
- Sales staff are sending out an old version of a product specification sheet
- You shipped product to a customer but sent it to the wrong address
- Your help desk did not respond to a customer within the 24 hour response time target
These issues could be identified through customer complaints, internal audits, external audits, incoming material inspection, or simply during normal testing and inspection activities.
Nonconformity is addressed with corrective actions and they are both in the same clause in ISO 9001:2015 (10.2).
ISO 9001:2015 no longer requires a documented procedure, but you must still keep records ('retain documented information') of the nonconformity and what was done to correct it.
You must establish a process (documented or not) for how your organisation will deal with non-conformance and how you will keep records of what happened.
If you make and/or sell goods, then you also need to work out how to deal with nonconforming product. The highest priority is to stop nonconforming goods reaching the customer, but the sooner you detect a problem, the less it will cost you.
In establishing a process for dealing with nonconformity you'll need to decide:
- who will determine what immediate actions will be taken to correct the problem, and what kinds of actions should be taken. These immediate corrective actions can be seen as 'damage control' and need to:
- stop further non-conformance
- contain the effects and stop any further processing of defective items- e.g. quarantine
- assess the effects of the problem - how much, how bad, what to do (e.g. scrap / rework),
- notify affected customers, if necessary
- how reworked items should be checked (if it is different from normal inspection)
- how and where a non-conformance should be recorded
- what steps should be taken to identify any defective product released to a customer
- what, if any, concessions/discounts will be given to the customer
- how a decision will be made on whether further corrective action is necessary
A basic process for manufacturing might go something like this:1. All staff are responsible for reporting a nonconformity to their supervisor.1. Staff or supervisor must fill out Form-99. *1. Also add details to the NCR register and record the assigned NCR number on Form-99. *1. Check any previous production to ensure conformity. Follow recall process if any defective product was released to customers1. Move all affected goods to the designated quarantine area and attach a 'non-conforming goods' label1. Investigate the source of the problem and correct it before resuming production.1. Supervisor to determine what action should be taken with nonconforming goods - rework, scrap, etc,1. Supervisor to record decision on Form-99. *1. If the scheduled delivery date will be affected, inform the customer1. Reworked items will be checked as per normal inspection with doubled sampling rates1. See corrective action for more details on what else needs to happen to address a nonconformity, i.e. actions to investigate and eliminate the root cause(s).1. All corrective actions to be recorded on Form-99. *1. When root cause has been addressed and verification of effectiveness has been completed, manager to review and sign-off NCR as closed on NCR register. *1. Manager and supervisor to review NCR register on a monthly basis and followup outstanding tasks.
* Toolbox software eliminates the need for either form or register, since both are taken care of within the software. Toolbox gives you a process to follow AND a place to keep the records for both non-conformance and corrective action.
In Toolbox software, nonconformities are recorded as 'Issues'. The same process is used to manage customer complaints, supplier problems, audit findings, improvement requests, maintenance issues, document change requests, and any number of other issue types you'd like to configure. Have a look in our user guide to see how you would report a nonconformance, and what the corrective action process looks like in Toolbox.
Introduction
Nonconformances occur when a service, product or process does not meet defined specifications or industry regulation and standards. Nonconformances negatively impact organisations in terms of cost, reputation, efficiency and effectiveness. Non-conformance management is a very useful quality tool in that it is a key Quality Management System (QMS) performance indicator and can quickly identify systemic issues within the organisation. Nonconformance management is the process whereby the organisation manages nonconformances such that they are promptly identified, documented, evaluated/investigated, segregated and dispositioned as per applicable regulations and standards.
A functioning nonconformance management system will bring many benefits to your organisation such as:
- Reduction in the occurrence and costly impact of nonconformances in terms of reputation, cost and resources
- Improvements to product/service and resultant customer satisfaction
- Efficient use of resources.
- Reduction in number of Customer Complaints
- Improved QMS effectiveness
It makes business sense to invest resources at the outset into designing a fit for purpose system that will deliver these benefits. If the nonconformance management system in your organisation is not fit for purpose it would be prudent to overhaul the system and redesign and the organisation will very quickly see the benefits.
What does a functioning non conformance management system look like?
A fit for purpose nonconformance management system is built on an well designed risk-based documented system that allows for effective implementation. Its foundation is the Plan Do Check Act cycle.
A functioning system is first and foremost reliant on everyone in the organisation engaging with the process which is underpinned by the culture of the organisation. Nonconformance management is not just Quality’s responsibility, all employees have a responsibility to report nonconformances and indeed potential nonconformances to responsible person(s) in the organisation. A blame culture within an organisation will impact negatively on the system as described below as it will result in under reporting of nonconformances and potential nonconformances.
Impact-Under Reporting of Non conformances
Non conforming service or product reaching the market place and associated consequences for the organisation
Non compliance with the process and resultant regulatory non compliance and related consequences.
Impact-Under Reporting of potential Nonconformances
Increase in number of nonconformances that occur and associated consequences.
Non compliance with the process and failure to meet an organisation’s requirement to implement continuous improvement
Failure to attain benefits of continuous improvements to product, service or process
Design, implementation and audit of a robust easy to use non conformance management system that allows for early detection, timely reporting, timely processing and accurate documentation of nonconformances is critical to its success.
A functioning nonconformance management system is in essence a series of building blocks namely design, documentation, implementation, audit, and continuous improvement cemented together with a clear understanding of the process and process objectives through training, communication and a supportive culture. Each of the nonconformance management system building blocks is described below. If any of the building blocks are missing or there is a disconnect then the non conformance process will not function.
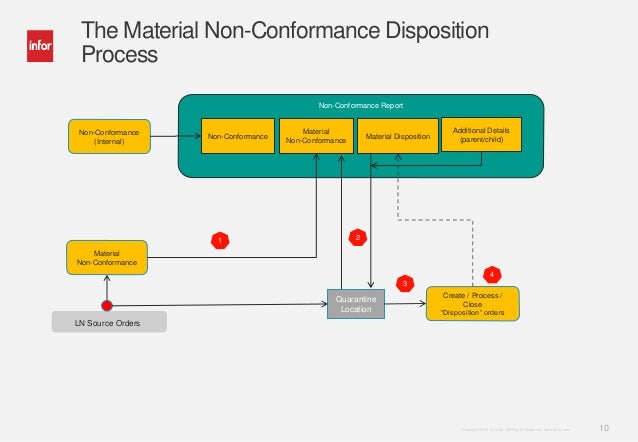
Supplier Non Conformance Procedure
Nonconformance management system building blocks
1. Design
The process design must be fit for purpose, the design of a nonconformance management process for a small organisation is very different to that required for a large organisation. The design must be continually reviewed for ongoing suitability as the organisation grows, the structure of the organisation changes or to meet changes to industry regulations and standards. The process should be designed by the process owner(s) who will be the subject matter experts. The design must be robust, easy to use and be clearly understood and supported within the organisation to ensure it is effective.
Non Conformance Procedure Food Industry
The process design should allow for categorisation of nonconformances as major or minor such that the Corrective/Preventive action (CAPA) is proportionate to the identified non conformance. Nonconformances should also be coded to allow trending of the data, code will identify the type of nonconformance i.e document, material, QMS and source of non conformance.
Design of quality record data templates is very important to facilitate the documentation process and ensure accurate recording of the nonconformance such that the cause of the nonconformance is correctly identified. If the cause of the nonconformance is not accurately identified then the correct CAPA will not be assigned to the nonconformance and will result in a reoccurrence of the non conformance and consequent waste of resources.
2. Documentation
The nonconformance system to be implemented within your organisation must be documented in a Standard Operating Procedure. The SOP must be clear, concise and unambiguous, the inclusion of a process flow chart is advised to aid understanding. A well written SOP will facilitate training and result in better compliance. All personnel must be trained in the SOP and training must be maintained. Documentation of a nonconformance must occur as soon as possible after identification of the nonconformance and contain as much detail as possible to facilitate reporting of high quality data and correct identification of the root cause of the nonconformance. Quality records generated from the process must be completed to the highest standard such that data integrity is achieved for subsequent trending of the data and accurate data analysis.
3. Implementation
A fit for purpose nonconformance management system is built on an excellently designed risk-based documented system that allows for effective implementation.
How do we effectively implement the nonconformance process?
- Process owner
- Clear Process Flows
- Training
- Timely actions
- Documentation
- Continuous monitoring
Implementation of the process is dependent on co-operation and communication between all stakeholders. A process owner should be assigned to oversee implementation and ensure all personnel are adhering to the documented process. The process owner will monitor implementation of the process from identification of the nonconformance through to close out of same and feedback into the process. Continuous monitoring of the process is essential to effective implementation. Weekly meetings with stakeholders where metrics for all nonconformances (Open, Closed, Closed Awaiting Verification) are discussed are a good continuous monitoring tool. The meetings can also be used to discuss CAPA’s, trends, process understanding and resource issues. Quality tools such as auditing and actively seeking feedback from employees are also very useful in monitoring effectiveness of the implementation of the process.
4. Audit
Regular auditing by personnel independent of the process is another very useful quality tool. Well executed audits can identify weaknesses and potential process improvements. Identification of weakness and correction of same within the process can result in prevention of nonconformances and associated impact in terms of reputation and costs. Identification and implementation of improvements to the process may also result in a reduction of number of nonconformances. Audits are also needed to ensure compliance with the process, measurement of process efficiency and to report back into the QMS. Audits of a process can also facilitate resource planning.
5. Continuous Improvement
Continuous improvement of the process will result in a more efficient system and consequently a reduction in the number of nonconformances and enhanced benefits of a functioning non conformance management system for your organisation. Continuous improvement is achieved by analysis of outputs from audits, CAPA’s and feedback from employees. Data analysis of these sources is used to identify process improvements and any systemic issues in the organisation which are addressed through update of the process and delivery of the benefits of the improved process.
Conclusion
Non Conformance Procedure Template
Investment in a well designed, effectively implemented and maintained nonconformance management system will bring many benefits to your organisation.
Non Conformance Procedure Sample
Contact Real Regulatory to help you design a bespoke non conformance management and your organisation will very quickly reap the benefits of the investment.